Gut microbes and diabetes
It seems that every day we are discovering more about the role of microbes to our health. We really have to revise our definition of what a human (or any other animal or plant) is: we are not just a creatures of 10,000,000,000,000 cells containing the DNA we got from mother and father. We have 10 times that many cells which are microbial, and we are only now beginning to understand how profoundly they affect us.
Together with obesity, insulin resistance is the harbringer of metabolic syndrome. Insulin resistance is when the body cannot use insulin effectively. Insulin is needed to help control the amount of sugar in the body. As a result, blood sugar and fat levels rise. Therein lies the path to morbid obesity, diabetes, stroke, and heart problems.
So what’s the connection of metabolic disease to bacteria? Well, for one thing, we know that in obese people the bacterial population in the gut is different, and the different population of bacteria may lead to a vicious cycle contributing to obesity.
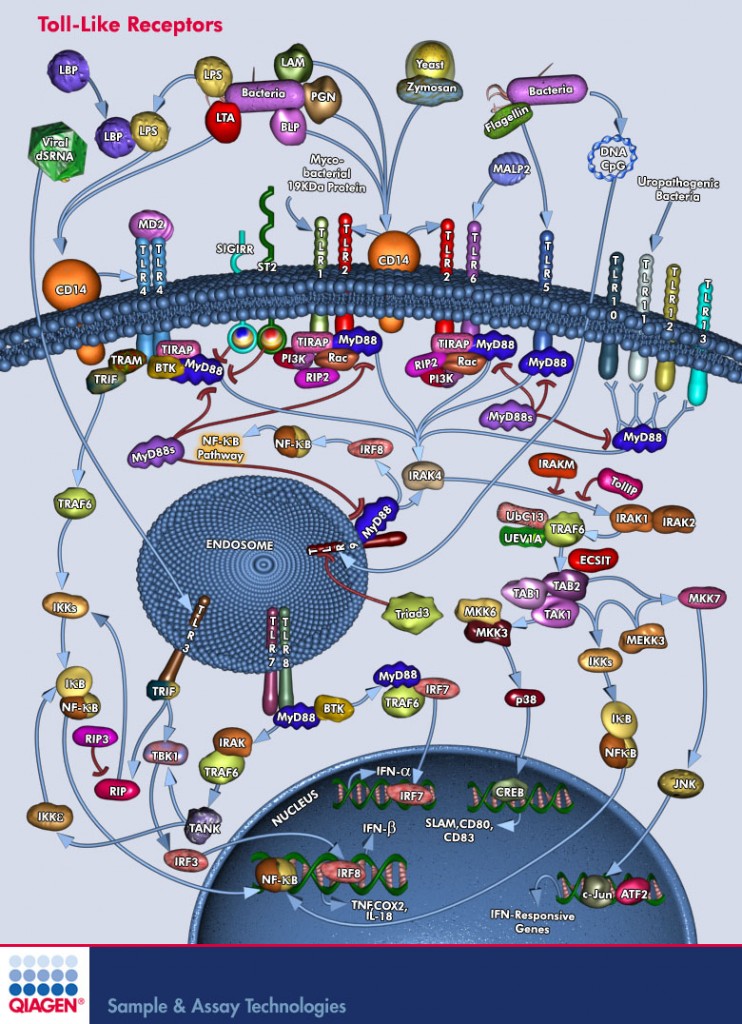
Another possible connection has to do with an important group of molecules in our body called Toll-like receptors, or TLRs. TLRs are a family of membrane proteins that sense a wide variety of bacterial populations and activate our innate immune system. TLRs are like a first-defense warning station: they sense the bacterial enemy first, and, if needed, activate the proper defense mechanisms. Researchers studying TLR-2 have created knockout mice lacking TLR-2, and they discovered is that many of TLR-2 knockout mice do not develop insulin resistance when fed with a high-fat diet. Think about it: all the McCrap you can eat, yet your blood sugar level remains normal (although you still grow fat). So why does that happen? How come these mice lacking a bacterial sensor also seem immune to insulin resistance?
To answer this, we must understand that TLR receptors (quite a few are known so far) are known to serve as a bridge (or “mediate crosstalk”) between the immune system and the body’s metabolism. Mice without TLR-5 develop eating disorder known as hyperphagia which is characterized by an increased appetite; they also show other pre-diabetic symptoms: hypertension, high lipids, and insulin resistance. I have posted before about how TLR-5 may control the type of gut bacteria mice have and, in turn, control their propensity for obesity.
TLR-4 deficient mice, on the other hand, seem to be protected from insulin resistance, just like TLR-2 deficient mice. So a connection between these front-line sensors of the immune system and whole body metabolism is well-known.
A group of researchers from Brazil have decided to look further into these “diabetes resistant” mice. The thing about mutant TLR deficient mice, is that they are normally grown in sterile conditions because possible infections and because the uncontrollable gut microbes add uncontrolled variables to any experiment. When scientists cannot precisely control for experimental conditions, they face two choices: One, they can deviate from the model to emulate “real world” better, but sacrifice control of one or more of the variables in an experiment. Or, maintain full control of the experiment and sacrifice a simulation whatever they are trying to model. Most scientists go with the second option: they would prefer to have a well-controlled model, even if it supposedly detracts from its supposed practicality and application to “real life”. That is because a model (in our case, mutant TLR mice), is somewhat removed from the real thing anyway: mutant TLR-deficient are basically a an artificial construct used to investigate the effect of knocking out a TLR from the mouse, so hopefully we can draw conclusions about humans. So the second type of possible error is the one scientists generally prefer to make.
But Andrea Caricilli and colleagues have decided to look at TLR-2 knockout mice in non-sterile conditions. Rememebr: TLR-2 knockouts seem to have protection from insulin resistance. What Caricilli and her colleagues discovered was quite the opposite of what was known so far: TLR-2 knockout mice were not protected from insulin resistance. Quite the opposite: the mutant mice developed metabolic syndrome. But did the gut bacteria do it? To check that, the researchers treated the mice with broad-spectrum antibiotics for 20 days. After that, the bacterial species that re-colonized the mice’s guts were quite different in their composition from the bacterial species that originally inhabited them. And they did not have meteabolic disease, or the symptoms were much less severe.
So here it is: changing the mice’s gut microbiota changed them from mice with insulin resistance to mice without insulin resistance. Yes, there are mutant mice, but still: insulin resistance was turned off by changing the types of microbes in the gut.
They then transplanted the microbiota from TLR-2 mutants which had insulin resistance to regular mice. And what do you know: the regular mice then showed symptoms of insulin resistance and metabolic disease.
There is a lot more to this paper than these two experiments: they have also investigated many other parameters, trying to come up with the chain of events that bacteria trigger when causing metabolic disease. I won’t get into that, the paper is quite long with some 20(!) figures. A lot of work went into this. But the bottom line again supports what has been shown in other studies: the bacteria that live in our gut are responsible for our metabolism, and it is the interaction between the bacteria and our immune system that not only protects us from pathogens, but also protects us (or not) from metabolic disease.
 Update: this post has been slashdotted. Exercise extreme caution.
Update: this post has been slashdotted. Exercise extreme caution.
Caricilli, A., Picardi, P., de Abreu, L., Ueno, M., Prada, P., Ropelle, E., Hirabara, S., Castoldi, A., Vieira, P., Camara, N., Curi, R., Carvalheira, J., & Saad, M. (2011). Gut Microbiota Is a Key Modulator of Insulin Resistance in TLR 2 Knockout Mice PLoS Biology, 9 (12) DOI: 10.1371/journal.pbio.1001212



















I certainly hope useful therapies are developed from this research. I’ve long wondered about the influence of gut microbes on diabetes. After a being prescribed long courses of a couple of antibiotics to combat a bout of diverticulitis, I went through a period of time when my blood sugar levels were very near normal and very easy to control (even when I ate more and–admittedly–less healthy food). Some years later, however, my blood sugar levels (and insulin resistance) began to climb again. When I was prescribed those antibiotics, I was told they would kill off all my gut bacteria (good and bad), so they placed me on a lactobacillus acidophilus supplement to help rebuild my gut biota. In light of my personal experience, this research is *extremely* interesting.
I was wondering how the high levels of antibiotics in animal feed would effect the gut bacteria in humans. Could it lead to the chronic obesity seen in today’s youth? How much antibiotics are present in today’s meat?
Shawn: great question. I am not sure if anyone looked at that. I guess that an epidemiological study is warranted. For example, comparing mostly beef & pork consumers (where antibiotics in the feed are legal, at least in the US), and poultry consumers (which are mostly antibiotic free). However, it would be very hard to separate other factors, such as overall calorie intake (higher in beef & pork than in poultry), associated lifestyle, etc. Perhaps someone else can chip in with suggestions?