Blood, sweat and spit
A short follow up to the previous post on latherin. A quick reminder: latherin is a protein that exists in the horse’s sweat and saliva. In the sweat, latherin acts like a detergent, wetting the horse’s coat to allow for better water evaporation and hence better cooling. In the saliva, it helps wet the horse’s dry feed, aiding digestion. It’s an interesting example of a protein performing different physiological functions in different contexts.
Widdowquinn made an interesting observation about our tendency to color a protein with a function of our liking. Quoting the comment: “Is the ‘function’ of latherin to aid heat transfer, or digestion, or both? Or does it make no sense to imbue the protein with any such ‘function’ – only to note that it is able to act in both these ways (potentially among others)“.
This observation is very true and is interesting especially with latherin. Latherin is part of a large group of evolutionarily related proteins containing a domain known as Bactericidal permeability-increasing protein (BPI) / Lipopolysaccharide-binding protein (LBP) / Cholesteryl ester transfer protein (CETP). The observed common function of all proteins that have this domain is that they bind fatty molecules (lipids) that constitute the cell membrane. The differences lie in the context of which lipids they bind and what happens as a consequence. For example, BPI proteins serve as potent antibacterial agent, binding lipopolysaccharide (LPS), a bacterial toxin expressed on the outer layer of the bacterial membrane. LPS causes a severe inflammatory response when in the blood stream, but BPI, secreted by our immune system, dampens down the response, and also kills the bacteria it binds. LBP also binds LPS, but acts as an alarm system, increasing the inflammatory response. Another family similar proteins is found on the lung surface, and are also a line of defense against bacteria, by similar mechanism. This is the Palate, Lung, and Nasal epithelium Carcinoma associated protein (PLUNC). The names is scary, but it was given in the context of their discovery, cancer research. PLUNC family proteins are used as cancer markers, but cancer has nothing to do with their primary function. BASE is another interesting and puzzling homolog, which may be a “dying gene”: expressed in a small quantity, but does not seem to be producing a viable well-folded protein product. It is expressed in mammary glands, and in saliva. Sounds familiar? Remember that mammary glands are evolutionarily modified sweat glands: BASE is also used clinically as a marker for breast cancer.
CETP has nothing to do with bacterial membranes; rather, it transports a precursor of cholesterol, which is a building block for animal membranes.
So we see here an interesting case of functional radiation: while the different proteins in the family maintain very similar sequences, their functions differ in physiological context, and also in organisms which express them (even bacteria use an LPS against other bacteria!), and the tissues that express them.
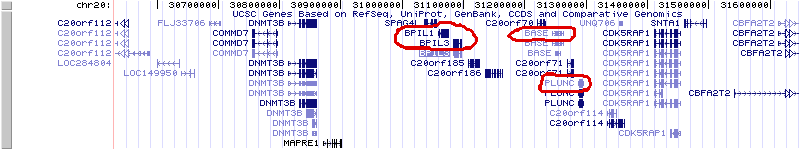
Moreover, with the exception of CETP, the genes coding for those proteins are clustered together in the same region in chromosome 20. This indicates that they are not only homologs (i.e. arose form a common ancestor) but paralogs: homologs that have arisen due to gene duplication. Gene duplication is a potent evolutionary mechanism for acquiring new functions: while the ancestral gene continues to do its work, the duplicate, which is redundant, has less selective pressure on it, can may adopt other functions. We also see a duplication in the same chromosomal neighborhood of the latherin gene in the horse, but only two, or possibly three neighboring paralogs.
Latherin and a neighboring homolog in horse chromosme 21. Click to enlarge. If you like playing around with the UCSC genome browser, try finding the third.
So we went from horse sweat, to bacterial defense, to a dying gene in human which is very much alive and lathering in horses. Ah, the zigzagging wonders of protein evolution! Was latherin initially a bacteria-killing protein that just happened to work well as a sweat enhancer? Possibly, seeing that even bacteria have a BPI domain. It might even still serve in a capacity as an immune defense protein, both in the sweat glands and in the salivary glands. Yes, we do color genes with the brush we happen to hold in our hand at the moment, but it seems like nature uses many different brushes, and it’s fun to try and find them all. (Does this metaphor even make sense?)
Disclaimer: genomic map pictures were generated using the fantastic UCSC Genome Browser. No horses or unicorns were harmed during the making of this post.
Bingle, C., & Craven, C. (2004). Meet the relatives: a family of BPI- and LBP-related proteins Trends in Immunology, 25 (2), 53-55 DOI: 10.1016/j.it.2003.11.007
Bingle, C. (2004). Phylogenetic and evolutionary analysis of the PLUNC gene family Protein Science, 13 (2), 422-430 DOI: 10.1110/ps.03332704




















Thanks for the post on latherin, I must say I learned a few things from this post.
Nick
I’m delighted to have helped provoke this post: the revolution against teleology in annotation starts here! 😉
I don’t really want a revolution – teleology has its place. I’m with you that it’s fun to try to work out what potential functional capacities genes are able to encode. My ‘go away and stop asking’ answer when asked a protein’s function is that it’s the complete set of its possible interactions in all contexts – which might be true, but is not a useful description 😉 It’s essential to human understanding that we simplify our descriptions of those capabilities, even sometimes to the point of caricature, just so that we can have a handle on them. And particularly when working out what a system might do, a teleological point of view can help. In practice, we just have to get in the functional ballpark and refine our descriptions from the most likely inferred behaviours in a sensible context, keeping in mind that the situation is complex, and that our descriptions are almost certainly incomplete, and potentially inaccurate. This is possibly how we’ve evolved to see and categorise the world in general, so maybe it’s actually more efficient for our own understanding to behave like this, despite the inevitable errors and philosophically questionable perspective?
Codifying this uncertainty and flexibility in computational terms, though – that’s another question entirely. We don’t do badly as a science, considering the difficulty.
Meanwhile the gene products evolve on, fitting as well as they are able into their shifting environment and corresponding selection pressures, visiting bacterial defence and surfactant properties, ignorant of our attempts to pigeonhole them…