Repost: a very loose and circular association to Pi Day
(Originally published March 14, 2009)
Happy Pi (π) Day! Americans write dates in the MM/DD/YYYY format instead of the DD/MM/YYYY format used by the rest of the world. Usually a rather painful and confusing format if you did not grow up with it, causing checks to bounce and leases to expire for those who recently moved to the US, but it has a few benefits: you can take the numeric representation of March 14, and you have the first three digits of Pi. This coincidence is good enough to celebrate a day around the uber-celebrity of numbers. (Heh, I said “around”). Everybody’s welcome.
This is the day all geeky bloggers come out and try to: (1) show how smart they are; (2) connect Pi, usually in some improbable and tenuous fashion, to whatever theme they have in their blogs and (3) try to make an original observation of pi no one else has made before. So that is exactly what I am going to do today.
Sort of.
Well, probably not.
Smarts
Well, I remembered Pi day, didn’t I? OK, that does not show I’m smart, just shows my brain is a repository of useless trivia. Look at the time of publication of this post: March14, 1:59am which is 3.14159. Hey, five digit time stamp that’s smart! (Not very original though, also I’m actually up at this time finishing a grant proposal).
A post with a less than tenuous connection to Pi
Some virus capsids are icosahedral. Not really spherical but sort-of. Bacteria have flagella motors that are circular. Micelles are usually spherical. Microvesicles are spherical. All these are a good start for pi-topics.
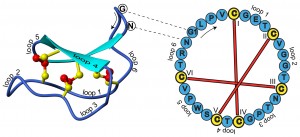
Well, too bad. I actually want to write about circular proteins. Only “circular” in this case does not mean “circle shaped”: hence, we are chucking Pi out the window right now. Stick around though, these proteins are really cool.
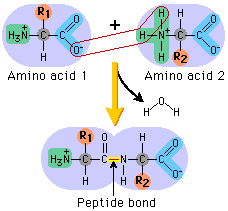
You were probably taught that proteins are linear chains of amino acids that fold into a shape that produces their function. The links connecting the chains are peptide bonds. But there is no real reason why the carboxy terminus (right side) and amino terminus (left side) would not bond themselves. It just has never been observed, or looked for. Well, they do. And some proteins are circular, like a snake biting its own tail.
These cyclotides are very robust. For one, they are almost immune to proteases: enzymes that break up proteins. Many proteases attack the edge of the protein (exoproteases, because they start from the “outside”), but there are no edges to attack here. The disulfide bonds, their short length make them immune to endoproteases as well as to heat, pH, etc.
What do cyclotides do?
They protect the organism that produces them. All kingdoms of life produce cyclotides, everything from bacteria to Rhesus monkeys. (Actually, I am not sure about Archaea). Cyclotides seem to act in different mechanisms: some form holes in the membrane of the attacking microbe; plant cyclotides stunt the growth of feeding caterpillars. Interestingly, the same plant peptide, Kalata B1 induces uterine contractions in mammals. This is how it was discovered: a physician working in the Democratic Republic of Congo noticed that laboring women were drinking tea made from Oleanda affinis to induce childbirth. Theactive ingredient was the first cyclotide to be discovered. Since then, cyclotides have been shown to be antibiotic, antiviral and insecticidal.
Do humans produce cyclotides?
I could not find anything about that in the literature. So I took the amino acid sequence of a recently discovered monkey cyclotide, rhesus theta defensin 1 (RTD1) sequence and BLASTed it (TBLASTN: protein vs. nucleotide) against the human genome. No results. Of course, this 5 minute trial proves very little. TBLASTNing short sequences (the RTD1 is only 18aa long) is a bit sticky. If you are a beginning bioinformatics student looking for a course or rotation project, finding candidate Cyclotides in humans (or in other genomes) might be a good idea. There are about 100 known sequences, so quite a bit for a training set to start from. You can build a profile or an HMM, and do some more sensitive searches.
But what about Pi?
Sigh.. well, here is an XKCD oldie but goldie nerd litmus test… enjoy…
Trabi, M. (2002). Circular proteins — no end in sight Trends in Biochemical Sciences, 27 (3), 132-138 DOI: 10.1016/S0968-0004(02)02057-1
PELEGRINI, P., QUIRINO, B., & FRANCO, O. (2007). Plant cyclotides: An unusual class of defense compounds Peptides, 28 (7), 1475-1481 DOI: 10.1016/j.peptides.2007.04.025
Wang, C., Hu, S., Martin, J., Sjogren, T., Hajdu, J., Bohlin, L., Claeson, P., Goransson, U., Rosengren, K., Tang, J., Tan, N., & Craik, D. (2009). Combined X-ray and NMR analysis of the stability of the cyclotide cystine knot fold that underpins its insecticidal activity and potential use as drug scaffold Journal of Biological Chemistry DOI: 10.1074/jbc.M900021200
Comments are closed.