The workings of a cellular water pore, and something about obesity
Maintaining a water balance is essential to life. Cells must regulate their water content carefully and within a very narrow margin. Too much water intake, and the cell bursts like a water balloon; too much water outflow, and it shrivels like a raisin.
The cell itself is contained in a waterproof membrane. But there are gateways in that membrane, to import solutes and food, extract waste and also maintain a water and electrolyte balance. One way to maintain a water balance is the aquaporin: a protein complex running through the membrane that lets water flow into the cell, in a controlled fashion Aquaporins are essentially very narrow tubes, the width of the tube is the width of a single water molecule.
How is this water pore controlled?
Two groups at the University of Gothenburg in Sweden and at the Max Planck institute in Goettingen, Germany have solved the structure of yeast aquaporin 1 Aqy1 at a resolution of 1.15 Å (1.15×10-10m), enabling them to resolve water molecules and positions of atoms in the protein at a very rare clarity. They reported their findings in June’s PLoS Biology.
Gerhard Fischer, Urszula Kosinska-Eriksson and their colleagues discovered that on the side of the aquaporin that is in the cell there is a rather elaborate gating mechanism to control water influx. Just how elaborate is not exactly clear. The end of the protein chain forms a complex termed “helical bundle” with a Tyrosine residue in the channel, which blocks the water flow.
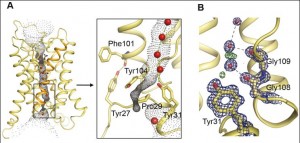
A: General view of the view of the yeast aquaporin, with the single-molecule-wide water channel in the middle; B: a close up showing the constriction near the end of the channel, and the gating mechanism. Click to enlarge. doi:10.1371/journal.pbio.1000130.g002
Aqy1 is actually a tetramer — four protein pore molecules arranged together in the membrane, all facing the same direction. So it’s more like a sheaf of four tubes.
A brief animation from the Protein Data Bank showing the four subunits together from different angles. (You need Java to see this, takes a bit of time to download and start).
Fischer and Kosinska-Eriksson also suggest the possibility of other control mechanisms. First, by phosphorylation: binding organic phosphate molecules to proteins is a common control mechanism in the cell, and a way to pass on signals. they found that when a certain well-located amino acid that can be phosphorylated is replaced by one that cannot be phosphorylated, the ability ot regulate water flow is hampered. Another suggestion they have is mechanosensitivity, or membrane movement,such as occurs during environmental stress. It is known that Aqy1 plays a role in cold shock: when the yeast is exposed to freezing temperature. There is definitely much more to learn about how aquaporins are controlled, triggered, and blocked.
Here is an animation of another aquaporin, Escherichia coli‘s aquaglyceroporin GlpF, showing water molecules (red & white spheres) moving through the channel. It was created by the Theoretical and Computational Biophysics Group at the University of Illinois Urbana-Champaign. Red is negative charge, blue is positive. Look for the yellow sphere about 1/3 form the top… (outside the cell) it eventually reaches the bottom (inside the cell), but that takes a while, due to Brownian motion. Electrostatic charges along the pore eventually force an overall one-way traffic into the cell.
The animation was created using VMD (they say that at the end credit, but that is a very short frame, and hard to catch).
You can read more about aquaporins on the aquaporin page of the Theoretical and Computational Biophysics Group. The page has great graphics, animations, and a high resolution version of the movie, and much more information about different types of aquaporins, and how they control the cell’s water content.
Aquaporins have also been implicated in obesity. Mice that had the gene for aquaporin-7 knocked out have started to accumulate fat at the age of 12 weeks, and become heavier than their counterparts: a mouse form of adult onset obesity. The reason is that aquaporin-7 is expressed in fat cells and this particular aquaporin also transfers glycerol. Glycerol is a small three-carbon carbohydrate synthesized in fat cells from glucose as a building block for to fat molecules. Aquaporin-7 acts as a glycerol pressure valve, letting some of it back into the bloodstream. If the aquaporin-7 is missing, glycerol accumulates in the fat cells, forcing the cell to synthesize more fat form this basic fat building block. The fat cells become larger, and the animal bearing them – fatter. This has been known for four years now, but I haven’t seen any recent progress on this aspect of obesity, at least not through an (admittedly cursory) scan of PubMed.
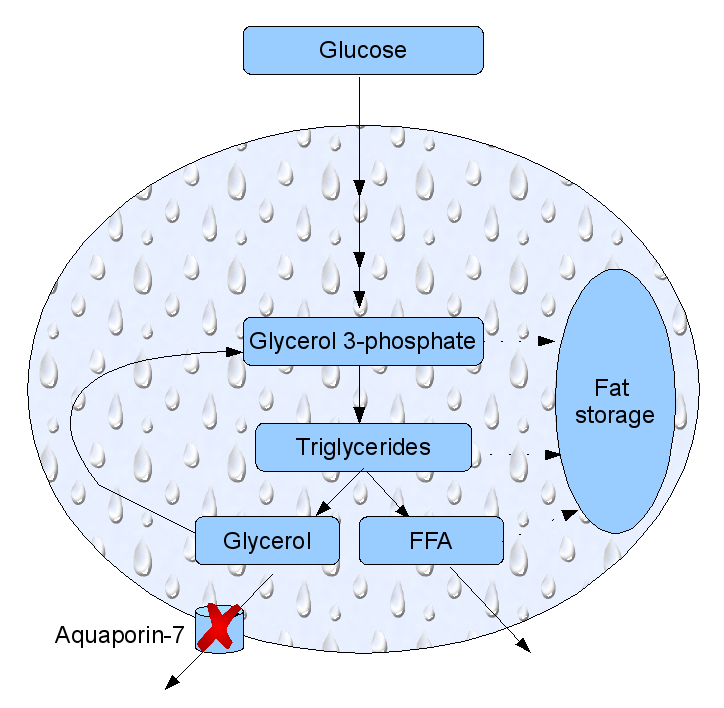
Fat cell metabolism. Glucose may end up as glycerol. If aquaporin-7 is not expressed, excess glycerol get stored as fat. FFA: free fatty acids. After Gema Frühbeck Nature 438, 436-437 (24 November 2005)
Fischer, G., Kosinska-Eriksson, U., Aponte-Santamaría, C., Palmgren, M., Geijer, C., Hedfalk, K., Hohmann, S., de Groot, B., Neutze, R., & Lindkvist-Petersson, K. (2009). Crystal Structure of a Yeast Aquaporin at 1.15 Å Reveals a Novel Gating Mechanism PLoS Biology, 7 (6) DOI: 10.1371/journal.pbio.1000130
Tajkhorshid, E. (2002). Control of the Selectivity of the Aquaporin Water Channel Family by Global Orientational Tuning Science, 296 (5567), 525-530 DOI: 10.1126/science.1067778
Frühbeck, G. (2005). Obesity: Aquaporin enters the picture Nature, 438 (7067), 436-437 DOI: 10.1038/438436b
Comments are closed.


















